| Extinction Coefficient | 46 100 M-1 cm-1 at 260nm |
| Molecular weight (free acid) | 1578.3 g/mol |
| Molecular formula (free acid) | C50H63N20O30P5 |
| Salt | Na+ |
| Form | 100 mM water soutions at pH 6.5 |
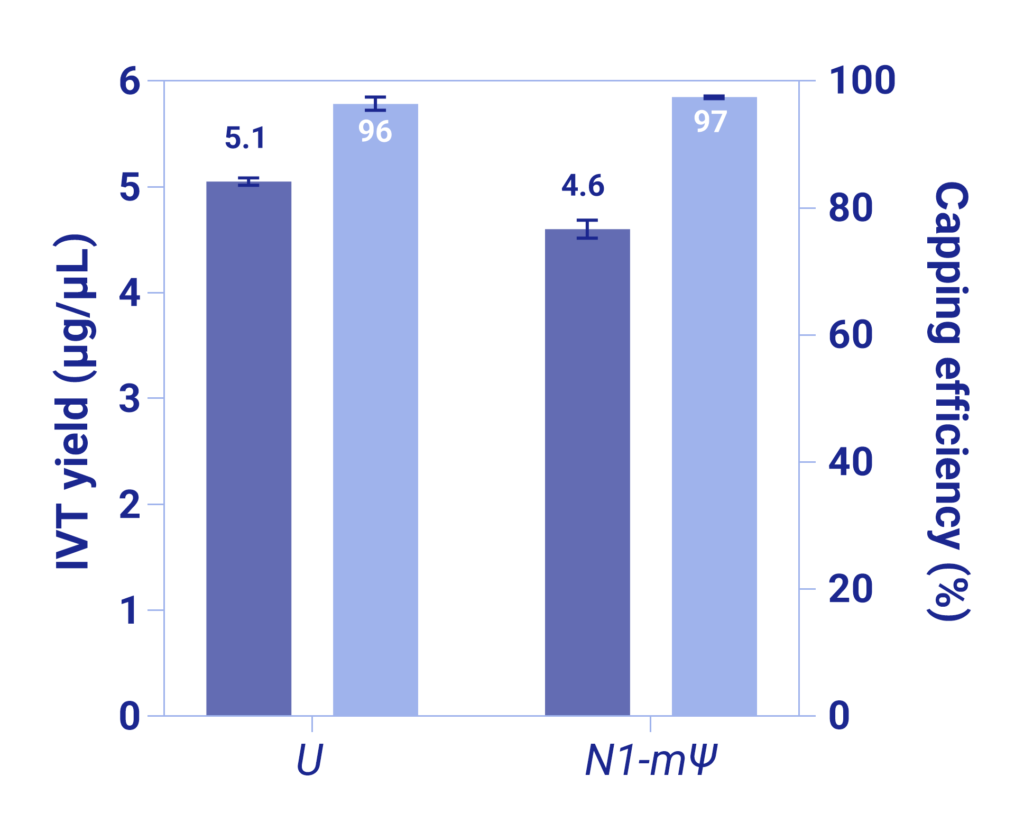
We are proud to introduce a new line of ExploRNA’s cap analogs: AvantCap® Q – an innovative solution design to simplify and boost your IVT process.
AvantCap® is the name of our flagship product, featuring unique benzyl modification that enhances its biological properties. Q stands for Quatre – tetranucleotide cap analogs facilitating better alignment with the DNA template ensuring high capping efficiency.
AvantCap® Q offers both, Cap 1 (AvantCap® Q1) and Cap 2 (AvantCap® Q2) versions, tailored to meet your specific needs.
AvantCap® Q2 is fully compatible with plasmid templates containing T7 polymerase φ6.5 promoter starting with AG.
mRNA: firefly luciferase; N1-MeΨ | Transfection: Lipofectamine | Timepoint: 4h | Caps: Cap1, AvantCap variants | Cell types: HEK293T, human and murine macrophages

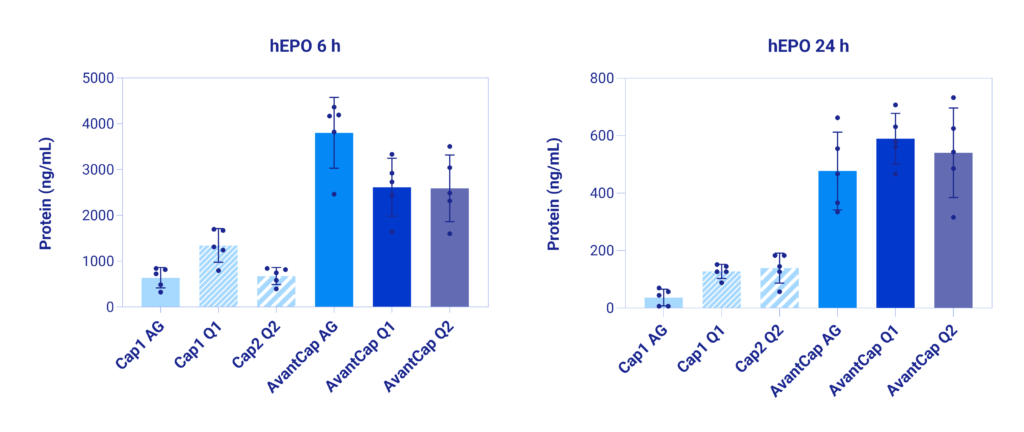
mRNA: human EPO; unmodified U | Dose: 1 µg | Caps: AvantCap variants and their unmodified analogs | LNP: clinical grade | Administration: intravenous